Introduction
Crimean-Congo Hemorrhagic Fever (CCHF) is a potential high-threat zoonotic disease caused by the Crimean-Congo hemorrhagic fever virus (CCHFV), a tick-borne virus belonging to the Nairoviridae family, genus Orthonairovirus [1,2]. The disease is characterized by the abrupt onset of high fever, headache, myalgia, gastrointestinal symptoms, and in severe cases, hemorrhagic manifestations, multi-organ failure, and death. With a case fatality rate estimated between 10% and 40%, CCHFV is among the most virulent of hemorrhagic fevers affecting humans [3,4]. The World Health Organization (WHO) has designated CCHFV as a priority pathogen and a target of global epidemic preparedness initiatives due to its growing geographical distribution, severe clinical outcomes in humans, potential for nosocomial outbreaks, and lack of mitigation strategies [5,6].
Transmission of CCHFV occurs primarily through bites of infected Hyalomma ticks or via direct contact with the blood or tissues of infected animals or humans [7]. Livestock, such as cattle, goats, and sheep, can be infected and while livestock mainly experience asymptomatic infection, they are key species in the transmission cycle of the virus to other hosts and humans [8]. Populations at highest risk for disease transmission include farmers, herders, veterinarians, abattoir workers, and butchers, who may encounter infectious materials from animals or humans, or tick infestations through routine animal husbandry or slaughter activities [9,10]. This occupational risk is exacerbated in low- and middle-income countries, where protective equipment, training, and veterinary oversight are often limited.
The Democratic Republic of the Congo (DRC), the second-largest country in Africa in terms of land area, encompassing vast ecological zones that are favorable for maintaining both tick populations and supporting livestock production. Agriculture and animal husbandry comprise a significant portion of the country’s informal economy and livelihood base, particularly in rural areas [11]. The DRC has a fragile public health infrastructure, limited veterinary surveillance, and lack of routine diagnostic capacity that has hampered efforts to monitor or quantify zoonotic diseases such as CCHF. Since the virus was first described and documented in Crimea in the 1940s and first virus isolation in DRC in the 1950s, epidemiological data regarding the scope of CCHF disease risk in central Africa remain sparse and fragmented [12]. To date, there has been no comprehensive One Health assessment of CCHFV prevalence among Congolese livestock and persons who work in close contact with livestock.
This study presents a cross-sectional assessment of CCHFV seroprevalence and risk factors associated with occupational and environmental exposures among livestock (cattle and swine), persons with occupational exposure to livestock including farmers, herders, and abattoir workers. This is among the first integrated One Health studies of CCHFV prevalence in the DRC which links animal and farmer serosurveillance with farm management practices and vector distribution. The results from this study could be used to inform both national policy and international preparedness frameworks. Moreover, they may help to close a critical knowledge gap in an under-studied region of sub-Saharan Africa and to illustrate how strategic global health engagement aligns with national security objectives. The proactive detection of zoonotic viruses, such as CCHFV, at their source contributes not only to the protection of vulnerable populations in endemic areas but also to safeguarding the health and stability of nations far beyond their borders.
Results
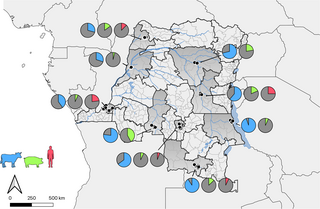
Among the 1,118 cattle surveyed across nine provinces 57.0% (95%CI: 54.1-59.9%) were seroreactive. Cattle from two provinces in the southeast, Tanganyika and Lualaba, had 94.6% (95%CI: 89.9-99.2%) and 90.7% (95%CI: 84.9-96.5%) reactivity, respectively. Among the 1,020 swine surveyed 13.4% (95%CI: 11.1-15.2%) were seroreactive. Swine from Kasai province had 42.1% (95%CI: 32.2-52.0%) seroreactivity, the highest rate of seroreactivity among swine (Fig 1). Attached ticks were found on 592 (52.9%) of all cattle and 4 (0.4%) of swine at the time of collection; seroreactivity was not associated with the presence of ticks (Table 1). Among the collected ticks, 16 Hyalomma rufipes were identified on 9 cattle from four provinces: Kinshasa (6), Sud-Ubangi (1), Kasai (1), and Tanganyika (8). Among these 9 cattle, 6 were seroreactive for CCHFV. Very few (<1%) cattle and swine were experiencing illness or exhibiting symptoms at the time of data collection. Two other tick genera we identified: Amblyomma (n = 1401, 91.4%) and Rhipicephalus (n = 115, 7.5%).
Of the 180 agricultural workers surveyed, 12.8% (95%CI: 7.9-17.6%) (23) were seroreactive for CCHFV antibodies. Maniema and Kinshasa provinces had the greatest number of seroreactive individuals, 8 (25%, 95%CI: 10.0-40.0%) and 6 (26.1%, 95%CI: 6.9-39.3%), respectively. Among all participants, 65.6% reported that they had contact with a sick animal in the past year. While there was a slightly greater proportion of seroreactivity among those who had contact compared with those who did not (15.2% vs 8.5%), the relationship was not statistically significant (chi-sq p = 0.2060). The use of personal protective equipment (PPE) was limited as 80% reported that they rarely or never wear PPE. 55% of participants reported that they live on the farm property. Across the cohort, 16.1% reported that they never wash their hands during their workday, 35.6% reported handwashing once or twice daily. Outside of work, 86.1% of agricultural workers reported that they entered the forest, 34.4% reported hunting wild animals, and 53.3% reported eating bushmeat (Table 2).
Of the 56 cattle farms visited, all but one farm in Kinshasa province had at least one seroreactive animal. On average, 59.5% of cattle tested at each facility were seroreactive to CCHFV; at seven farms, three in Lualaba province and four in Tanganyika, all animals tested were seroreactive. Among the 65 swine farms visited, 56.9% (37) had at least one seroreactive animal on the day of sampling. On average, we observed 21.5% seroreactivity among selected swine among farms that had at least one seroreactive animal.
All but three (94.5%) cattle farmers reported that they allow their cattle to graze and 87.5% reported that they had an issue with ticks within their herd. Among swine herds, 16.9% allow grazing and 3 farms (4.6%) reported tick problems. During collection it was observed that pigs were often kept in small, wet enclosures unsuitable for ticks compared with grassier and more open areas where cattle were kept. For both cattle and swine, the use of vaccines was less common, 19.6% and 21.5% of farms, respectively; however the use of any kind of veterinary medicine was very common—73.2% of cattle farms and 67.7% of swine farms regularly used and had access to medicines. When asked about nose-to-nose contact with other species, rats (37.5%), mice (28.6%), and poultry (28.6%) were most common among cattle herds. Among swine herds, 55.4% of farmers reported nose-to-nose contact with rats and mice. Swine farms with at least one seroreactive animal reported much higher rates of contact with rodents compared to those that did not— for rats: 66.7% vs 33.3%, Chi-sq p = 0.0314; for mice: 69.4% vs 30.6%, Chi sq p = 0.0083 (Table 3).
Discussion
This survey represents the largest integrated One Health CCHFV serologic survey in the DRC to date. In this paper, we focus on a defined cohort of agricultural workers with direct livestock contact, paired with concurrent sampling of cattle and swine—the first reported CCHFV seroprevalence data for swine in the DRC. In addition to serology, we collected and identified attached ticks, enabling integration of vector ecology with host infection status. Detailed farm management and biosecurity practices, along with participant behavioral data (PPE use, hygiene practices, wildlife contact), allowed us to identify modifiable on-farm and individual risk factors. Together, these features provide a high-resolution, One Health perspective on CCHF disease risk in DRC.
The DRC has two seasons—dry and rainy. Due to logistical constraints and the size of this serologic study, Equateur, Kasai, Lualaba, Maniema, Sub Ubangi, and some Kinshasa sites were visited during the rainy season; Kasai Oriental, Tanganyika, Tshopo, and some Kinshasa sites were visited during the dry season. Previous work in sub-Saharan Africa and Iran have demonstrated that precipitation has a negative association with tick abundance and may impact CCHFV prevalence in the environment [15,16]. While Lualaba was visited during the rainy season and Tanganyika during the dry, CCHFV seroreactivity among cattle and swine in this southeastern region was quite high, nearly ubiquitous on some farms. This may be due to the savannah-mosaic grasslands which provide a more suitable habitat for ticks in comparison with forested regions of the country. Spatial analysis suggests that the risk of CCHF disease occurrence is higher in arid and semi-arid lands [15]. Indeed, in Uganda seroprevalence surveys of CCHFV in livestock have found rates of seroreactivity to be very high (~90%) [9,17]. In Tanzania, which neighbors Tanganyika, fewer seroprevalence studies have been conducted but a 2016 study found 50% seropositivity in cattle [18]. Further models on CCHFV serosurveillance and environmental factors are needed to explore this spatial relationship.
While the observed seroreactivity indicates a high potential for CCHFV transmission among livestock and humans, only 16 Hyalomma rufipes were identified from ticks found on nine cattle. While Hyalomma ticks are considered the primary vector for CCHFV, the other two tick genera we identified Amblyomma (n = 1401, 91.4%) and Rhipicephalus (n = 115, 7.5%) can also transmit the virus, and these species were found in abundance [19–21]. Detection of the presence of CCHFV among collected ticks from seroreactive livestock can provide further understandings of CCHFV carriage among tick genera. Indeed, 87.5% of farms reported tick infestations among their herds. All collected attached ticks were homogenized, and following RNA extraction (QIAGEN), tested for CCHFV with commercially available RT-PCR kits. All measured cycle threshold (Ct) values exceeded established validated assay cut offs and thus were considered negative for CCHFV.
We observed a statistically significant relationship between swine farms with a seroreactive animal and the rate of nose-to-nose contact between the herd and rodents. On cattle farms this relationship was not significant but around a third of farms reported rodent contact. While rodents are not considered a primary reservoir for CCHFV they may play an indirect role in the transmission chain as a host for the tick vector, an amplifier, or bridge host between questing ticks and livestock [22,23].
Interestingly, we did not observe a pattern between human and cattle seroprevalence. In Lualaba, where 90.7% of cattle were seroreactive, just 6.5% of samples agricultural workers were seroreactive. Further scrutiny is needed to understand the causal pathway of CCHFV infection in agricultural workers and the role that livestock play as intermediate vectors. In general, we did observe that the agricultural workers surveyed likely experience frequent low-level exposures during their job duties and lifestyles outside of work that have an additive effect over their lifetimes. Handwashing and PPE use were limited, and a majority of individuals interacted closely with sick animals. We observed a greater proportion of seropositivity among those aged 50 years or older compared with younger age groups which may indicate that there is repeated CCHFV exposure and risk across the lifecourse. Seroreactivity was not associated with any reported zoonotic exposures: visiting the forest, hunting wild animals, slaughtering or butchering wild animals, or preparing wild animals to eat. However, 80% of participants reported that they entered the forest regularly for personal reasons, indicating that environmental and zoonotic exposures are commonplace across the DRC. Further laboratory work to detect active or recent infection is needed to further understand the risks of CCHFV transmission in agricultural environments.
Two prior DRC serosurveys reported lower—or highly variable—anti-CCHFV IgG seropositivity. In 2013, a ruminant survey in southeastern DRC detected 2/514 seropositive cattle (≈0.4%) [24]. A subsequent analysis of archived sera collected in 2017–2019 from humans and cattle, tested with the same in-house NP-ELISA used here, found 4.4% seropositivity in humans and 42.8% in cattle [25]. Differences between those estimates and ours likely reflect geography, sampling frame, host species, exposure risks, timing/seasonality, and assay performance. Longitudinal studies, coupled with entomological sampling, are needed to clarify trends in livestock infection and tick infection rates. Assay choice is also pivotal: performance varies across platforms—for example, a 2017 Ugandan study reported 12.6% seropositivity using an in-house assay versus 75% with a commercial IDVet ELISA [26]. While the in-house assay used here performed well compared to a widely used commercial kit, harmonized assays and standardized cut-offs will be essential for future CCHFV serosurveillance. Additionally, while the assay primarily detects IgG, it may also detect other immunoglobulin isotypes with affinity for Protein A/G in some species.
Overall, this One Health cross-sectional survey found the exposure to CCHFV over the life course of cattle to be greater than anticipated and reinforces WHO’s designation of CCHF disease as a priority pathogen for endemic preparedness. This survey was cross-sectional, and as such represents only a snapshot of potential CCHF disease risk in the DRC. Further longitudinal research can provide important understandings of CCHF disease risk over time. As only 16 Hyalomma ticks were identified, targeted research of questing ticks, rather than feeding ticks could provide important data on tick habitat in the DRC. Additional testing on collected ticks could provide further understandings of CCHFV transmission in agricultural settings.
The DRC occupies a strategically important position for zoonotic surveillance. It is home to a high density of human-livestock interaction, emerging land use change, climate-sensitive vector habitats, and limited healthcare access—all contributing factors to pathogen spillover risk. Agricultural workers are at heightened risk for zoonotic transmission and proper health reporting systems and communication regarding occupational safety should be prioritized to support CCHFV control and outbreak preparedness.