Introduction
Burkholderia pseudomallei is a Gram-negative saprophytic bacterium commonly isolated from soil and water in tropical and subtropical endemic regions. It is the etiologic agent of melioidosis, a potentially fatal disease that is often underdiagnosed or overlooked in areas with limited diagnostic capabilities or low public awareness [1–2]. Human melioidosis is primarily acquired through the subcutaneous contact, inhalation, or ingestion of B. pseudomallei, while human-to-human or animal-to-human transmission is rare [3]. The severity of melioidosis is influenced by factors such as exposure to large inocula of B. pseudomallei, infection by highly virulent strains, and host comorbidities such as diabetes and chronic kidney disease [1,4].
In endemic regions such as Ubon Ratchathani in northeastern Thailand, Darwin in the Top End of the Northern Territory in Australia, and Kaohsiung in southern Taiwan, B. pseudomallei has been consistently detected from soil, which serves as a natural reservoir for the bacterium [5–7]. Climatic changes, in particular, have been shown to be associated with the emergence of melioidosis [8–9]. Correlations between the presence of B. pseudomallei in the environment, the incidence of melioidosis in humans and animals, and seropositive rates in local populations have been documented [10–12]. To date, the classification of melioidosis-endemic regions has been primarily based on the environmental isolation of B. pseudomallei and the occurrence of non–travel–associated clinical cases in tropical and subtropical areas [13–14].
The high environmental isolation rates of B. pseudomallei highlight the need for increased clinical awareness of melioidosis in regions where the disease may occur. Nonetheless, systematic investigations into the environmental distribution of B. pseudomallei remain incomplete in many regions, including parts of Taiwan [7]. Furthermore, the vertical distribution of B. pseudomallei in soils has been reported down to 30–90 cm, and even as deep as at 100–200 cm below the surface, challenging the likelihood of frequent human exposure [7,15,16]. Comparing isolation rates across heterogeneous soil, geographic locations, and time periods reveals that detecting B. pseudomallei at the surface remains challenging, even with established consensus protocols for environmental sampling [17–18].
To enable the rapid analysis of large numbers of soil samples, PCR-based techniques were developed and applied for the specific detection and quantification of B. pseudomallei in environmental surveillance [7,19,20]. Challenges remain, as soils with high PCR-positive rates did not consistently yield B. pseudomallei isolates [7,20], suggesting that unidentified factors may influence changes in the bacterial population during cultivation. Addressing this issue could help bridge the gap between PCR detection and successful bacterial recovery.
From 2003 to 2024, a total of 790 melioidosis cases have been reported in Taiwan, with 82.6% of cases occurring in southern Taiwan, which is recognized as an endemic area due to higher incidence rates and the presence of B. pseudomallei in the environment [21]. In contrast, only five cases of melioidosis have been reported in eastern Taiwan, a region with limited healthcare infrastructure and no prior investigations into the environmental distribution of B. pseudomallei. This study aims to explore the geographical distribution of B. pseudomallei reservoirs in cropped soils across Taiwan, assess the vertical distribution of the bacteria in PCR-positive locations during a rainy event, and identify potential biological factors that may explain the failure to isolate the bacterium from PCR-positive soil samples during environmental surveys.
Materials and methods
Ethics statement
All procedures were approved by the Institutional Biosafety Committee of Kaohsiung Veterans General Hospital (VGH-1081226–1); and National Kaohsiung Normal University (NKNU-18/23–001).
Microorganisms
B. vietnamiensis NKNU04, B. multivorans NKNU07, B. cenocepacia NKNU05, and B. pseudomallei VGH27 used in this study were isolated from the environment and identified using biochemical and molecular methods (see below). Viable B. pseudomallei were handled in an airflow-controlled laboratory (BSL-3 level, at Kaohsiung Veterans General Hospital).
Random sampling strategy
The random sampling aimed to identify the locations of B. pseudomallei soil reservoirs across Taiwan. Sampling was conducted using Geographic Information System (GIS) data along the public road network, maintaining a straight-line interval of 0.3 to 3 km. Sampling sites were selected in agricultural land, while urban areas, protected zones, undisturbed lands, and restricted-access areas were excluded. Sampling took place from October 2022 to March 2023, a period of relatively stable precipitation in Taiwan. Considering that the 0–45 cm soil layer is disturbed by tillage practices, sampling was conducted to a depth of 60 cm in rice fields, herbaceous plant areas (e.g., vegetables and commercial flowers), and fallow land (typically covered with grass) to ensure the soil remained undisturbed and had not been recently exposed to sunlight. In fruit tree orchards, sampling was conducted to a depth of 30 cm, as tillage is not required.
At each sampling site, three individual holes were dug 1–5 m apart and 500 g of soil was collected from each hole. The digger (10 cm in diameter) was disinfected with 70% alcohol between collections to prevent cross-contamination. Soil samples were placed in sterile bags and promptly transported to the laboratory, where DNA extraction was carried out in the following days. Sites positive for B. pseudomallei DNA were defined by the presence of a positive PCR result in at least one of the three holes (see DNA extraction and PCR methods below).
Fixed-interval and vertical sampling
Based on the PCR-positive results from the random sampling strategy described above, fields were selected from the northern (Cluster 1), central (Cluster 5), southern (Cluster 7), and eastern (Cluster 8) regions of Taiwan (see Fig 1 for cluster positions). To facilitate sampling, surface vegetation was gently cleared, and the ground was lightly leveled to ensure consistent soil access across sites while minimizing disturbance to subsurface layers. Sampling sites were determined using GIS and arranged in fixed-interval grids of 0.64 m2 within a 64 m² field, resulting in a total of 100 sampling squares per field. Sampling was conducted four times: following an initial sunny day, a rainy day, and then the first and second subsequent sunny days (which may not have been consecutive). A sunny day was defined as having no rainfall, a UV index >7, and a sunshine rate >35%, while a rainy day was defined as having rainfall >40 mm, a UV index <7, and a sunshine rate <35% in this study. All sampling was conducted from July to September 2023. Climatic information was obtained in real time from a climate station near the sampling field (https://codis.cwa.gov.tw/StationData?target=station; stations 467050, 467480, 467441, and 467660).
The sampling sites were selected using a random strategy along the public road network. Symbols indicating plantation types, sampling sites and PCR-positive results are shown in the upper-left corner. The cumulative incidence of melioidosis (2003–2024), and the PCR positivity rate (October 2022–March 2023) are presented. Asterisks (*) above a number indicates sites selected for vertical distribution analysis in different regions. The basemap was adapted from open-access shapefiles provided by the Taiwan Government Open Data Platform (https://data.gov.tw, Open Government Data License, version 1.0).
https://doi.org/10.1371/journal.pntd.0013640.g001
Soil samples (10 g for isolation; 2 g for DNA extraction) were collected from the land surface (0–10 cm, as sun-exposed soil), as well as from depths of 30 cm (disturbed soil) and 60 cm (undisturbed soil) below the surface. Additionally, backup soil was collected at each sampling site and stored at room temperature (70% humidity) for future bacterial community structure analysis. Isolation of B. pseudomallei and total DNA extraction were performed immediately after soil collection (see Isolation and PCR methods below). Culture-negative for B. pseudomallei was defined as the failure to yield the bacterium in three independent isolation attempts.
Bacterial Community Structure Analysis Sampling
Following the results of a fixed-interval survey of the southern land surface (Cluster 7) on a rainy day, bacterial community structure analysis was conducted based on the following findings: a PCR-positive soil sample that did not yield B. pseudomallei at (6,4), a PCR-positive site with culture confirmation at (6,5), and a PCR- and culture-negative site at (7,4), according to x-y coordinates (see S1 Fig for coordinates). To minimize the impact of prior sampling, each new sampling point was located 10 cm from the center of the previous hole, and 500 g of soil was collected per site. Before bacterial community analysis, the PCR and culture results of the soil samples were reconfirmed.
DNA Extraction from environmental samples
Soil samples (2 g or 10 g), collected through random or fixed-interval strategies or for bacterial community structure analysis, were suspended in sterile water at a 1:1 ratio and placed on a rotator (120 rpm) at 42°C overnight. The following day, 1 mL (from 2 g samples) or 5 mL (from 10 g samples) of homogenized mixture was inoculated into 10-fold volume of Ashdown’s broth (10 g/L Trypticase soy broth, 4% (v/v) glycerol, 5 mg/L crystal violet, 50 mg/L neutral red, and 8 mg/L gentamicin) and incubated for 2 h (for all environmental surveys) or 36 h (only for bacterial community structure analysis).
Soil pellets from Ashdown’s broth cultures were obtained by centrifugation at 16,000 × g for 10 min at room temperature using an Eppendorf centrifuge (Eppendorf, Hamburg, Germany). DNA was then extracted from the pellets using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Briefly, 0.25 g of soil pellets was subjected to chemical and mechanical lysis in a zirconium bead tube containing lysis buffer. Cell disruption was performed by vortexing using a bead tube adapter. Inhibitory substances were removed using proprietary commercial reagents, and nucleic acids were bound to a silica membrane using a chaotropic binding buffer. After a two-step wash, genomic DNA was eluted in 10 mM Tris buffer and stored at –20°C.
Cultures, PCR, qPCR, and Biochemical Identification
Approximately 10 g of soil was suspended in 10 mL of sterile water and placed on a rotator (120 rpm) at 42°C overnight. The following day, 5 mL of homogenized mixture was inoculated into 50 mL of Ashdown’s broth at 42°C for 7 d. Streaking cultures or plating counts with serial dilution (n = 3, each culture) on Ashdown’s medium were performed daily for up to 7 d. Each suspected colony was cultured in Luria-Bertani (LB) broth at 37°C for 18–24 h and then subjected to biochemical identification, DNA purification, and PCR amplification.
For molecular identification, the DNA was extracted from bacterial pellets obtained from LB broth cultures after growth from a single colony, using the DNeasy Blood & Tissue Kit (Qiagen), following the protocol for Gram-negative bacteria. Bacterial pellets, obtained by centrifugation at 16,000 × g for 10 min from overnight cultures, were subjected to enzymatic lysis, followed by proteinase K digestion and column-based purification. DNA was eluted in AE buffer (Qiagen) and stored at –20°C.
A B. pseudomallei PCR amplification targeting the 115-bp orf2 region of the type III secretion system (TTS1) was performed using purified DNA, with 45 cycles of 15 sec at 95°C and 15 sec at 59°C [19]. For quantitative PCR (qPCR), only purified DNA was used. The assay employed specific primers (BpTT4176F: 5′-CGTCTCTATACTGTCGAGCAATCG-3′; BpTT4290R: 5′-CGTGCACACCGGTCAGTATC-3′) and a fluorogenic probe (5′-CCGGAATCTGGATCACCACCACTTTCC-3′) in a final reaction volume of 25 µL. qPCR assays were performed using a 7900HT Fast Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA), following a previously described protocol [22].
Genomic DNA from B. multivorans, B. cenocepacia, and B. vietnamiensis was purified from suspected colonies grown in LB broth. Both full-length 16S rDNA (primer; F, 5’-AGAGTTTGATCMTGGCTCAG-3’; R, 5’-TACCTTGTTACGACTT-3’) and recA gene (primers; BCR1, 5’-TGACCGCCGAGAAGAGCA-3’; BCR2, 5’-CTCTTCTTCGTCCATCGCCTCA-3’) were amplified. The PCR conditions consisted of 35 cycles, with denaturation at 96°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 1.5 min [23]. Sequence analysis revealed >99.9% similarity to known sequences, leading to the identification of the bacteria as B. vietnamiensis NKNU04, B. cenocepacia NKNU05, and B. multivorans NKNU07, which were used in this study.
Biochemical identification of all isolates was performed using biochemical test profiles (API 20NE; bioMérieux, Marcy l’Etoile, France).
In the pre-test, over 96% and 100% of soil samples (n = 30, per endemic area) imported from melioidosis-endemic areas—Darwin in the Top End of the Northern Territory, Australia, and Ubon Ratchathani in northeastern Thailand, respectively—yielded B. pseudomallei isolates following the isolation protocols used in this study (S1 Table).
Bacterial community structure analysis
The bacterial community structure was analyzed using total DNA extracted after 2 and 36 h of incubation in Ashdown’s broth. A total of 36 samples were included for 16S rDNA sequencing, representing 6 individual soils across three soil categories: (1) PCR- and culture-positive, (2) PCR-positive but culture-negative, and (3) PCR- and culture-negative for B. pseudomallei, at both 2- and 36-h incubation time points. DNA quantification was performed with the Qubit dsDNA HS Assay Kit (ThermoFisher Scientific, San Jose, CA, USA), and quality was assessed with the Agilent 4200 TapeStation system (Agilent Technologies, Palo Alto, CA, USA), as part of services provided by Welgene Inc. (Taipei, Taiwan).
The 16S Long Read Kit (Loop Genomics, San Jose, CA, USA) was used to construct a full-length 16S rDNA amplicon library. Library sequencing was performed on an Illumina NovaSeq platform (Illumina, San Diego, CA, USA) in paired-end 150 bp mode. Raw short reads were processed on a cloud-based platform maintained by Loop Genomics, which assembled them into high-quality consensus contigs. Synthetic long-read (SLR) sequence data were analyzed using the Divisive Amplicon Denoising Algorithm (DADA2). Sequences were screened for the presence of forward and reverse primers and trimmed to appropriate full-length 16S rDNA sizes, ranging from 1400 bp to 1600 bp. Amplicon Sequence Variants (ASVs), representing exact and unique DNA sequences, were generated by DADA2 and used for analysis in this study. Heat Tree visualizations were created using an R-based program for each time point (2 and 36 h), with each tree representing a combined analysis of 6 individual samples per condition. Each sample was analyzed separately to generate ASV tables, and the merged output was used to construct the Heat Tree, allowing comparison of overall bacterial community composition across distinct conditions. Node colors represent sequence count abundance, and node sizes correspond to the number of distinct taxa.
Additionally, the top 14 populations (each comprising over 1% of the total community) were selected from the ASV tables to observe individual changes in total population at 2- and 36-h incubation across the following groups: PCR- and culture-positive, PCR-positive but culture-negative, and PCR- and culture-negative for B. pseudomallei (n = 6 per group × 2 time points).
Antagonistic assay
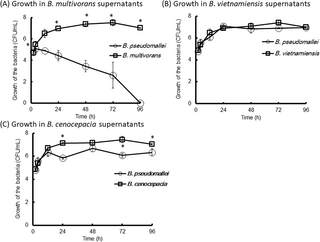
The supernatants of B. vietnamiensis NKNU04, B. cenocepacia NKNU05, and B. multivorans NKNU07 were obtained by culturing each strain in 50 mL of LB broth at 37°C for 48 h, followed by filtration through 0.22 μm membrane filters (Costar Inc., Cambridge, MA, USA). The incubation temperature was chosen based on the optimal growth rates of all tested strains. B. pseudomallei VGH27 was inoculated into the filtered supernatants at a final concentration of 5.5 × 104-1.0×105 CFU/mL and incubated at 37°C for 96 h (n = 6, per group). As controls, B. vietnamiensis NKNU04, B. cenocepacia NKNU05, and B. multivorans NKNU07 were inoculated into their respective supernatants in parallel experiments. Bacterial numbers (CFU/mL) were determined at defined intervals using serial dilution and plating.
Multilocus sequence typing (MLST)
The housekeeping genes (ace, gltB, lepA, lipA, nark, ndh, and gmhD) were amplified and sequenced using primers as previously reported [24]. The alleles at each locus were assigned by comparing the sequences with those available on the website (https://pubmlst.org/). The STs (sequence types) were determined using the allelic profile database. Phylogenetic analysis was performed using MEGA11 (Maximum Likelihood Tree method; open source: https://www.megasoftware.net/) and the resulting tree images were generated using iTOL (branch lengths ignored; open source: https://itol.embl.de/).
Statistics
The descriptive statistic used for melioidosis in this study was cumulative incidence over the period 2003–2024. Spearman’s rank correlation was used to assess the relationship between PCR positivity and soil depth, as well as the inverse association between B. pseudomallei and other Burkholderia species during incubation. Correlations between PCR positivity at different soil layers and climatic factors—such as rainfall, sunshine rate, and ultraviolet index (UVI)—were evaluated using Poisson or negative binomial regression models, selected based on Akaike Information Criterion (AIC) values. Data from antagonistic assays were expressed as mean ± SD (standard deviation; n = 6 per group). Differences between groups were assessed using the Mann-Whitney U test. A p-value of <0.05 was considered statistically significant.
Discussion
In this study, we investigated the geographical distribution of B. pseudomallei, the causative agent of melioidosis, in soil samples collected across Taiwan. Melioidosis, a disease currently seeking classification as a neglected tropical disease by the World Health Organization (WHO), poses a significant threat to populations in low-income regions with limited medical resources [2]. The geographical distribution of B. pseudomallei in endemic areas is defined by regions with reported non–travel–associated melioidosis cases and culture-confirmed or PCR-positive detections in the environment [13]. In southern Taiwan, melioidosis hotspots have been identified based on a high number of reported melioidosis cases (12.14 cases per 100,000 people) and PCR- or culture-confirmed B. pseudomallei from soil or water samples [7,21]. In this study, we found that PCR positivity was 15.1% in southern Taiwan, significantly higher than in other areas. Although eastern Taiwan has reported only 0.95 cumulative cases per 100,000 people from 2003 to 2024, it has relatively limited medical resources. Notably, 16 out of 163 soil samples (9.82%) tested positive for B. pseudomallei in this region. These findings suggest that nearly 500,000 residents in the region may face underrecognized risks of exposure to the pathogen.
The geographical distribution of B. pseudomallei is notably uneven at the regional scale. However, within areas where it is detected, PCR-positive locations are consistently found in clusters ranging from 3.3 to 48.9 km². Cluster 6 is situated along the Er-Ren River in Tainan City, and Cluster 7 is near Kaohsiung City in southern Taiwan; both locations have reported melioidosis outbreaks [11,21]. Local acquisition of melioidosis has been reported from the environment, typically from soil or freshwater sources-even in regions with few cases, such as Mississippi, USA [25]. Moreover, B. pseudomallei can be washed out with eroded soil during rainfall and conveyed to remote areas [26]. In this study, a vertical distribution survey revealed high PCR positivity in the southern land surface (Cluster 7), increasing the risks of human or animal exposure to the pathogen. Specific climatic conditions, such as heavy rainfall or typhoons, can generate aerosols from the land surface, further facilitating airborne transmission [24,27]. According to climatic changes, the positivity rate of B. pseudomallei-specific DNA on the land surface increased on rainy days and decreased during subsequent sunny days. Bacteria with the same molecular STs were identified across three soil layers (60 cm, 30 cm, and land surface) during a rainy event. This finding suggests that rainfall facilitates the dissemination of B. pseudomallei from deeper layers to the land surface, but the bacteria do not persist on the land surface for extended periods under sunny conditions.
In crop fields, factors such as nutrient levels, soil types, iron content, and fertilization have been reported to partially influence the growth and survival of B. pseudomallei [16,21,28–31]. Variations in plantation practices across extensive field studies from high to low altitudes limit the ability to establish clear links among the presence of B. pseudomallei DNA, agricultural practices, and fertilization methods in this study. Although standardized protocols were used, the required clearing of vegetation and slight leveling may have disrupted the rhizosphere, as this microenvironment is sensitive to sampling disturbance and may have influenced our observations [32].
Notably, over 95% of positive sites were located below a latitude of 24.3°N, extending beyond the typical 20°N to 20°S range where melioidosis is most prevalent, in alignment with predicted distributions [13]. Although distinct regional genetic clusters were identified in this study, the genetic type ST99, identical to human isolates, was widely found from northern to southern regions, indicating that B. pseudomallei exists in the environment across Taiwan [33]. Human exposure to environmental B. pseudomallei depends on the likelihood of the pathogen appearing on the land surface under certain climatic conditions or at a depth of 30 cm due to agricultural activities such as plowing [5]. Several studies published between 2007 and 2015 reported the isolation of B. pseudomallei from southern Taiwan, suggesting that the bacterium has persisted in harsh soil environments in the region for many years [7,11,24].
A selective Ashdown’s medium containing antibiotics was developed to isolate B. pseudomallei from clinical specimens and was later applied to environmental isolation [17,34]. B. thailandensis is commonly found in endemic areas of Thailand, Sierra Leone, Nigeria, Malaysia, Myanmar, and Papua New Guinea [35–38]. B. humptydooensis, which is genetically close to B. thailandensis, has been isolated from the Northern Territory of Australia [39–40]. However, neither B. thailandensis nor B. humptydooensis DNA was detected in our environmental samples.
The rhizosphere-associated bacteria B. vietnamiensis, B. multivorans, B. cenocepacia, and B. pseudomallei are known to inhabit agricultural soils, standing water, sludge, waste, and polluted environments [41–45]. In our cultivation-based experiments, these four bacterial species became predominant, likely due to selective pressures-such as antibiotics or crystal violet-that suppressed the growth of other bacterial populations [34]. An inverse distribution pattern was observed between B. pseudomallei and gentamicin-resistant B. multivorans, B. cenocepacia, and B. vietnamiensis during incubation in PCR-positive Culture-neative groups. For these cultures, while B. multivorans, B. cenocepacia, and B. vietnamiensis maintained high population levels, B. pseudomallei declined to <0.04%, suggesting possible bacterial competition. Consistent with previous findings [46], the growth of B. pseudomallei was inhibited by the supernatants of B. multivorans in our in vitro assays. B. cenocepacia also showed mild inhibitory effects, whereas B. vietnamiensis had no significant impact.
However, the culture-based detection method used in this study—relying on Ashdown’s medium—selectively enriches for gentamicin-resistant bacteria and may not accurately reflect the true composition or relative abundance of Burkholderia species in soil. In vitro competition assays were performed under controlled laboratory conditions, which may not replicate the complex and variable interactions present in natural soil environments, such as nutrient gradients, microhabitat structures, and broader microbial community dynamics. Additionally, B. pseudomallei in soil may be predated upon fungi, amoebae, nematodes, and slime molds [47–50] and its growth may be suppressed by bacterial competition through mechanisms such as secretion of antimicrobial compounds, phage-mediated lysis, toxin–antitoxin systems, or Type VI secretion system (T6SS)-mediated killing [50–52].
The observed inhibitory effect on B. pseudomallei in the presence of filtered B. multivorans supernatants is likely due to secreted factors from B. multivorans, such as secondary metabolites, broad-host-range bacteriophages, or toxic molecules that impair B. pseudomallei growth [53–54]. These findings suggest that B. multivorans can exert an antagonistic effect on B. pseudomallei through the production of inhibitory factors, particularly under favorable environmental conditions. However, the exact mechanism underlying this inhibition remains to be determined. Further analyses, such as mass spectrometry (MS) or transmission electron microscopy (TEM) of the supernatants, are needed to identify the active components involved. Interspecies interactions among Burkholderia species warrant further investigation to better understand competitive dynamics within this genus in soil environments.
Beyond microbial factors, dynamic bacterial community structures can emerge under agricultural practices such as plowing and irrigation, as well as climatic factors like UV radiation, temperature, wind, and rainfall [55]. Climate change, in particular, has been shown to alter soil microbial communities and increase the risk of soil-borne diseases [56]. The occurrence and dissemination of melioidosis depend on the presence of environmental B. pseudomallei, particularly when it emerges in high abundance at the soil surface [24]. This disease is gradually spreading from endemic to non-endemic areas, often facilitated by flooding due to heavy rains [57]. Notably, melioidosis frequently reemerges in multiple regions following severe climate events and natural disasters [56,58]. Our finding that B. pseudomallei appears on the land surface during rainy periods offers a potential explanation for the observation that melioidosis outbreaks in Taiwan have typically followed heavy rainfall associated with typhoons [21].
In conclusion, our findings reveal that B. pseudomallei is distributed across Taiwan, including in regions with few reported cases, and that its surface emergence is strongly influenced by rainfall. The presence of competing soil bacteria, such as B. multivorans, may contribute to the underestimation of B. pseudomallei in culture-based environmental surveys, particularly in southern Taiwan.